by Erin Loury, Ichthyology Lab
Trying to tie scurvy-plagued sailors of yore to the field of marine biology might be a bit of a stretch, but I had such an awesomely geeky “Ah-ha!” moment regarding this classic maritime disease that I just had to share. (Plus I was reminded recently that we are fast approaching the 200th anniversary of Darwin’s birthday next year, so what better time to start with the throw-backs to 19th century explorers?).
My light bulb moment went something like this. Any school child could probably tell you that the disease scurvy (causing bleeding gums, spotty skin, and general nastiness) is caused by a vitamin C deficiency. We’ve all heard how things finally got better when those historic mariners sat around sucking limes. (Where is the team mom with those orange wedges when you need them?) But I never really understood how Vitamin C actually saves the day – until I came across the write-up of a biomolecular archaeology symposium in Science magazine (a U.K. researcher is finding some evidence for scurvy by looking at the proteins in 17th and 18th century sailor skeletons), and which explained the basic connection.
The answer, it turns out, is collagen – the most abundant protein in the human body. Twisted collagen fibers are in our hair, our tissues, even our bones. Anything that messes with this protein signals is bad news for the body – and scurvy is just that type of news. Collagen is chock full of proline, one of the of the amino acid building blocks that make up all proteins. So it stands to reason that anything that messes with proline messes up collagen, and before you know it, it’s a scurvy mess.
So what goes wrong? Well, some proline molecules need hydroxyl groups added to them to help reinforce structure of the whole protein molecule. A hydroxyl is simply an oxygen and hydrogen hanging out and sharing electrons. These hydroxyl groups don’t just appear on proline, enzymes have to put them there. And one of the cofactors (helpers) that these enzymes need to stick on the hydroxyl groups is – wait for it – Vitamin C!
There you have it – without the right tools (Vitamin C) to help you construct proper nuts and bolts (proline), your beams (collagen fibers) won’t be structurally sound.
And without a good framework, whatever you try to build is going to have some serious problems (like say, a human body). The builders of the Titanic should have known better than to go against this string of logic, just to throw in another maritime example…
So now when mom tells you to drink that orange juice, you can thank her for saving you from gum disease and further misery – and you can tell her how. Those poor sailors eating things like hardtack (which by all accounts, had the nutritional value of thinly disguised cardboard) didn’t stand a chance. But in scurvy’s defense: without it, Shakespeare would have been deprived of some of his choice insults. What do you say to that, you scurvy jack-dog priests?




Congratulations on the blog at school Erin! Keep us posted with reminders from time to time. We don’t want to miss anything. Love reading your articles as always. Love,
Aunt Marla
LikeLike
Your brainchild (blog) is fabulous. The article on scurvy is sooo interesting, and the video is great. Thank you, Erin.
Love, Grandma and Grandpa
LikeLike
Thanks, family, it’s nice to know that I will always be able to convince someone to read my writing! :) Even if the blog format is not the best hang-on-the-fridge material…
LikeLike
Great blog, Erin. Keep up the good work! I am glad to hear that you love what you are learning in marine biology.
Co Khanh
LikeLike
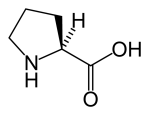
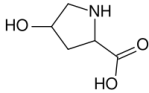
Regarding hydroxylation of proline and the stabilization.
In a recent article published in J. Am Chem Soc. Kotch et al. elucidated the mechanism whereby collagen stability conferred by proline hydroxylation. It turns out that hydration of the hydroxyl group is not the mechanism for the increased stability, but rather stereioelectronic effects of the OH group itself.
ARTICLE:
Stabilization of the Collagen Triple Helix by O-Methylation of Hydroxyproline Residues
Frank W. Kotch,†‡ Ilia A. Guzei,† and Ronald T. Raines*†§
Departments of Chemistry and Biochemistry, University of Wisconsin, Madison, Wisconsin 53706
J. Am. Chem. Soc., 2008, 130 (10), pp 2952–295
” The prevalent (2S,4R)-4-hydroxyproline (Hyp) residues of collagen are known to confer great stability upon its triple-helical conformation. The basis for that stability has been attributed to hydration of the pendant hydroxyl groups. Here, that attribution is shown to be incorrect. Replacement of the natural Hyp residues with synthetic (2S,4R)-4-methoxyproline (Mop) residues is shown by circular dichroism spectroscopy and differential scanning calorimetry to increase the conformational stability of the collagen triple helix.
Thus, the conformational stability conferred upon the collagen triple helix by O-methylation provides strong evidence that the hydroxyl group of Hyp acts primarily through stereoelectronic effects and that its hydration provides no benefit.
LikeLike
Highly energetic post, I enjoyed that bit. Will there be a part 2?|
LikeLike